Titration of Coca-Cola®
🔒 LAB RESOURCES:
Student Procedure [PDF] [DOC]
Teacher Annotated Procedure [PDF]
Complete Lab Guide [HERE]
GoogleSheet Data & Analysis [HERE]
SUMMARY/ OVERVIEW:
Students use an acid-base titration to determine the concentration of phosphoric acid ($\text{H}_3\text{PO}_4$) in Coca-Cola. By reacting the weak polyprotic acid with a standardized strong base ($\text{NaOH}$), the equivalence point is identified using a $\text{pH}$ meter or an indicator, allowing the calculation of the initial molarity of $\text{H}_3\text{PO}_4$ in the soft drink sample.
ESTIMATE TIME ⏰: 40-60 minutes
Preparation and Degassing (20-30 minutes)
Setup and Standardization (20 minutes)
Titration Trials (60 - 90 minutes)
SAFETY PRECAUTIONS:
Eye Protection is Mandatory: Always wear approved safety goggles throughout the entire experiment.
Handle Sodium Hydroxide ($\text{NaOH}$) with Care: The standardized $\text{NaOH}$ solution is a strong base and is corrosive.
Dispense $\text{NaOH}$ carefully from the buret. If contact with skin occurs, immediately flush the area with copious amounts of water for several minutes and notify the instructor.
Buret Safety: Handle the glass buret carefully to avoid breakage. Ensure it is securely clamped and that the tip is over the center of the flask during the titration to prevent spills.
Ingestion Prevention: Even though you are titrating a beverage, do not drink the Coca-Cola sample or any other chemical solutions in the lab. Wash hands thoroughly after the experiment.
Proper Waste Disposal: The titrated mixture contains neutralized acid and the titrant. Dispose of all chemical waste in the designated waste container as instructed by your teacher.
Background
The acid in soft drinks (aka "sodas" or "pops") provides a taste that is both sweet and sour, and does not interfere with other tastes in the drink. Phosphoric acid is the acid that is present in all soft drinks, but the percentage of phosphoric acid may vary (they don’t give out the secret recipes to just anyone!).
Pepsi contains citric acid and phophoric acid. Coca-cola only contains phosphoric acid. This lab determine the amount of phosphoric acid. It is also important that we do not use diet soft drinks since the artificial sweeteners that they contain have acidic functional groups that will also interfere with the titration.
Soft drinks are also carbonated beverages. The carbonation can produce some carbonic acid in the beverage, which would affect the results. Therefore, to ensure that you are only titrating the phosphoric acid you will use de-carbonated soda. This can easily be obtained by gently heating the soda, or as you may already know, by rapidly shaking the bottle — get all of the bubbles out!
You should remember from previous titrations that the titration is complete when you reach the equivalence point. The equivalence point is when starting material has completely reacted. Usually this is not visually apparent without special aid. In past experiments you probably used an indicator that changed color very close to the equivalence point to signal when the reaction is complete. This visual change is the endpoint. In this experiment, the Cola is dark brown and would mask any color changes thus preventing the use of such indicators.
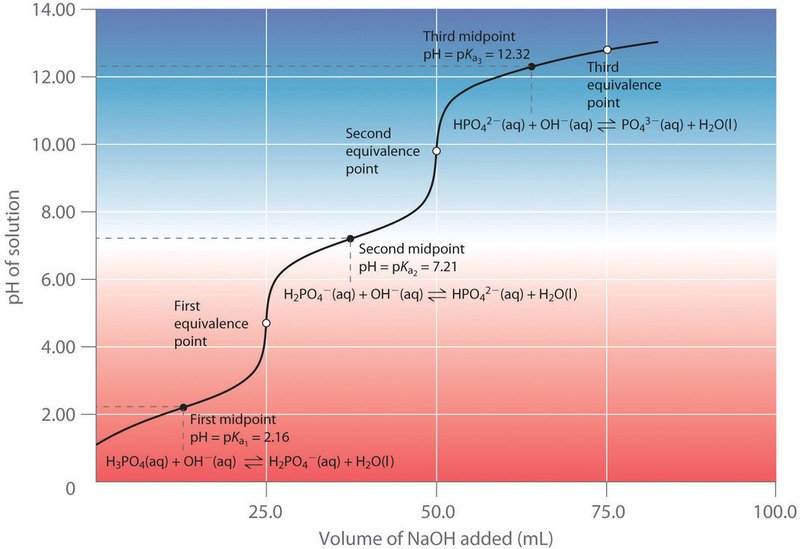
Phosphoric acid is a weak, triprotic acid. It dissociates as shown in Equations 1-3.
Phosphoric Acid ($\text{H}_3\text{PO}_4$)
Phosphoric acid is a weak, triprotic acid, meaning it can donate three protons ($\text{H}^+$) in a stepwise manner, each with its own dissociation constant ($\text{K}_{\text{a}}$):
$$\text{H}_3\text{PO}_4(\text{aq}) + \text{OH}^-(\text{aq}) \rightleftharpoons \text{H}_2\text{PO}_4^-(\text{aq}) + \text{H}_2\text{O}(\text{l}) \quad (\text{1st equivalence point, p}K_{\text{a}1} \approx 2.15)$$
$$\text{H}_2\text{PO}_4^-(\text{aq}) + \text{OH}^-(\text{aq}) \rightleftharpoons \text{HPO}_4^{2-}(\text{aq}) + \text{H}_2\text{O}(\text{l}) \quad (\text{2nd equivalence point, p}K_{\text{a}2} \approx 7.20)$$
$$\text{HPO}_4^{2-}(\text{aq}) + \text{OH}^-(\text{aq}) \rightleftharpoons \text{PO}_4^{3-}(\text{aq}) + \text{H}_2\text{O}(\text{l}) \quad (\text{3rd equivalence point, p}K_{\text{a}3} \approx 12.35)$$
In this lab, due to overlapping equilibrium and the very basic $\text{pH}$ required, typically only the first or second equivalence point is used for accurate analysis. We primarily titrate the first two acidic protons with the strong base ($\text{NaOH}$).
Interference from Carbon Dioxide ($\text{CO}_2$)
Soft drinks contain dissolved carbonic acid ($\text{H}_2\text{CO}_3$) formed from dissolved $\text{CO}_2$ gas. Carbonic acid is also an acid and will react with the titrant ($\text{NaOH}$), interfering with the determination of the $\text{H}_3\text{PO}_4$ concentration.
$$\text{CO}_2(\text{aq}) + \text{H}_2\text{O}(\text{l}) \rightleftharpoons \text{H}_2\text{CO}_3(\text{aq})$$
To ensure accuracy, the soft drink must be completely degassed (removing the $\text{CO}_2$) before titration, usually by gentle heating or prolonged stirring, so that only the phosphoric acid reacts with the $\text{NaOH}$ titrant.
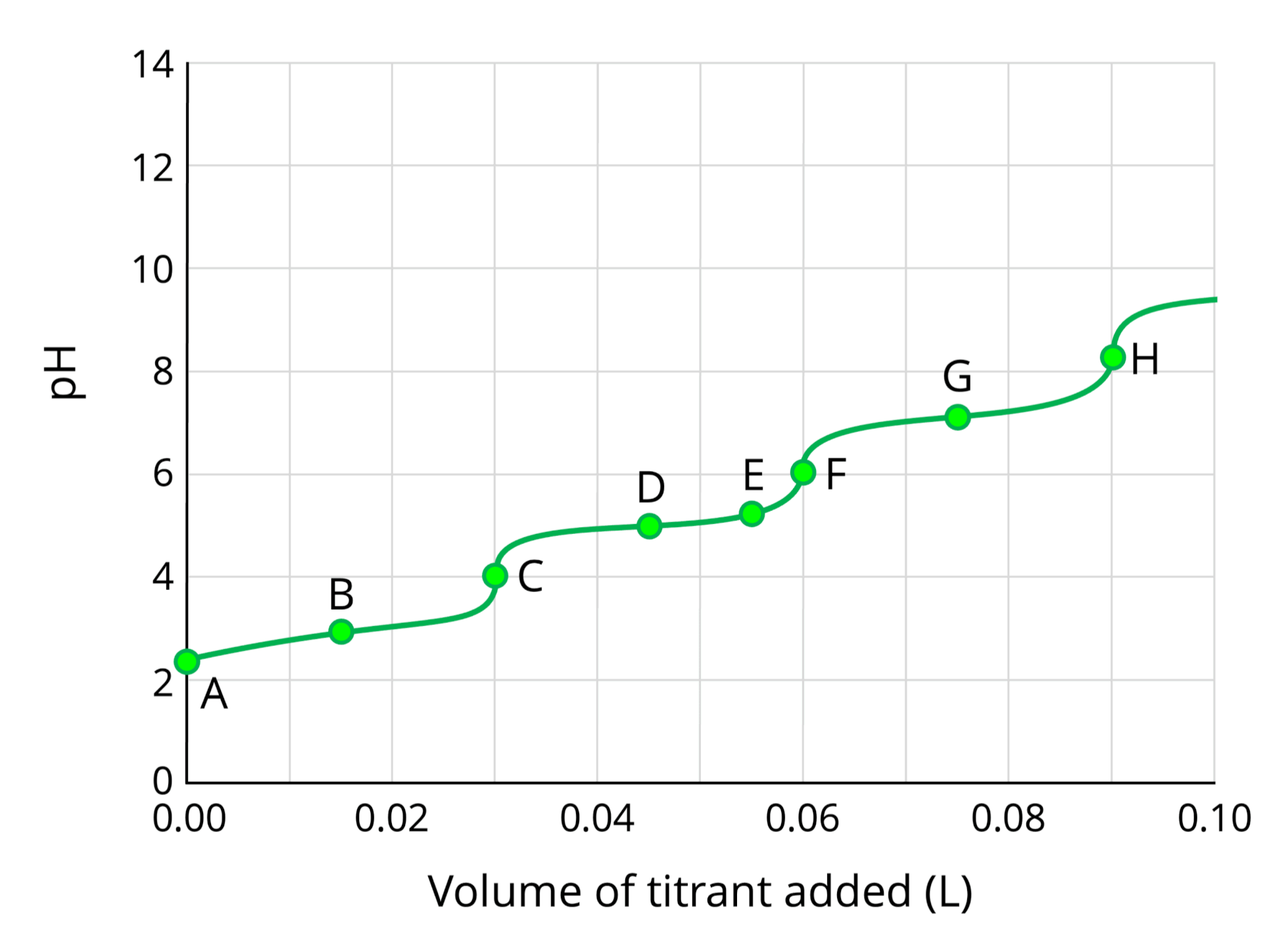
General Triprotic Titration Curve & Key Points
| Point | Description | Chemical Species Present |
|---|---|---|
| A | Initial Point | The solution contains only the starting acid, H3PO4. The pH is determined by the acid's initial concentration and its Ka1. | H3PO4 |
| B | First Buffer Region | The solution is a buffer containing a conjugate pair: the weak acid (H3PO4) and its conjugate base (H2PO4-). | H3PO4 and H2PO4- |
| C | First Equivalence Point ($V_{eq1}$) | All the initial H3PO4 has been converted to the intermediate species, H2PO4-. The pH is high because the solution acts as an amphiprotic salt. | H2PO4- |
| D | Second Buffer Region | The solution is now a buffer containing the weak acid (H2PO4-) and its conjugate base (HPO42-). | H2PO4- and HPO42- |
| E | Second Equivalence Point ($V_{eq2}$) | All the H2PO4- has been converted to HPO42-. This pH is typically near neutral. | HPO42- |
| F | Third Buffer Region | The solution is a buffer containing the weak acid (HPO42-) and its conjugate base (PO43-), which is a fairly strong base. | HPO42- and PO43- |
| G | Third Equivalence Point ($V_{eq3}$) | All the HPO42- has been converted to PO43-. The pH is very high because the PO43- ion is a strong conjugate base. | PO43- |
| H | Excess Base Region | Beyond the third equivalence point, the pH is determined almost entirely by the concentration of the excess strong base (OH-) added to the solution. | PO43- and excess OH- |