Podcast Episode
Companion Guides
Student/ Blank Guide
Use to follow along as your listen to the episode. Download pdf here
Teacher/ Annotated Guide
Use to follow along as your listen to the episode. Download pdf here
Essential Knowledge
◼ The mass spectrum of a sample containing a single element can be used to determine the identity of the isotopes of that element and the relative abundance of each isotope in nature. (SPQ-1.B.1) Go to Section
◼ The average atomic mass of an element can be estimated from the weighted average of the isotopic masses using the mass of each isotope and its relative abundance (SPQ-1.B.2) Go to Section
◼ The mass spectrum of a sample containing a single element can be used to determine the identity of the isotopes of that element and the relative abundance of each isotope in nature. (SPQ-1.B.1)
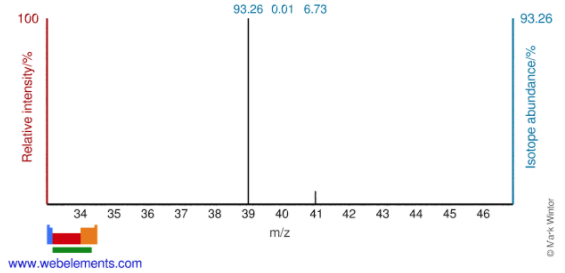
The mass spectrum of a sample of a pure element is shown below. Calculate the average atomic mass of the element and identify the element.
*** SEE BELOW FOR WORKED OUT SOLUTION ***
From the spectrum, I can see that there are three isotopes representing the following isotopes:
Mass: 39 amu, Abundance: 93.26%
Mass: 40 amu, Abundance: 0.01%
Mass: 41 amu, Abundance: 6.73%
I will find the weighted average of the isotopic masses using the formula below.
𝑨𝒗𝒆𝒓𝒂𝒈𝒆 𝑨𝒕𝒐𝒎𝒊𝒄 𝑴𝒂𝒔𝒔 = ∑ 𝒏 (𝒓𝒆𝒍𝒂𝒕𝒊𝒗𝒆 𝒂𝒃𝒖𝒏𝒅𝒂𝒏𝒄𝒆 𝒐𝒇 𝒊𝒔𝒐𝒕𝒐𝒑𝒆 𝒏) × (𝒎𝒂𝒔𝒔 𝒐𝒇 𝒊𝒔𝒐𝒕𝒐𝒑𝒆 𝒏)
𝑨𝒗𝒆𝒓𝒂𝒈𝒆 𝑨𝒕𝒐𝒎𝒊𝒄 𝑴𝒂𝒔𝒔 = (𝟎. 𝟗𝟑𝟐𝟔 × 𝟑𝟗 𝒂𝒎𝒖) + (𝟎. 𝟎𝟎𝟎𝟏 × 𝟒𝟎 𝒂𝒎𝒖) + (𝟎. 𝟎𝟔𝟕𝟑 × 𝟒𝟏 𝒂𝒎𝒖)
𝑨𝒗𝒆𝒓𝒂𝒈𝒆 𝑨𝒕𝒐𝒎𝒊𝒄 𝑴𝒂𝒔𝒔 = 𝟑𝟔. 𝟑𝟕𝟏𝟒 𝒂𝒎𝒖 + 𝟎. 𝟎𝟎𝟒𝟎 𝒂𝒎𝒖 + 𝟐. 𝟕𝟓𝟗𝟑 𝒂𝒎𝒖
𝑨𝒗𝒆𝒓𝒂𝒈𝒆 𝑨𝒕𝒐𝒎𝒊𝒄 𝑴𝒂𝒔𝒔 = 𝟑𝟗. 𝟏𝟑𝟒𝟕 𝒂𝒎𝒖
The average atomic mass of the element is 39.1347 amu.
Answer: The identity of the element is potassium, K. The calculated average atomic mass of 39.1347 amu is closest to the average atomic mass given on the periodic table for potassium.
◼ The average atomic mass of an element can be estimated from the weighted average of the isotopic masses using the mass of each isotope and its relative abundance (SPQ-1.B.2)
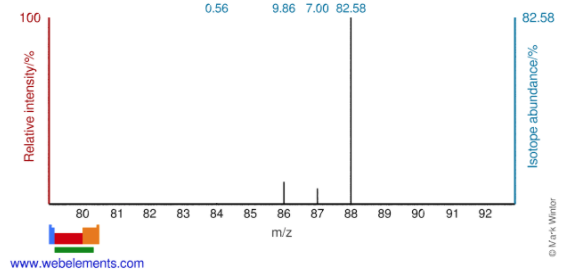
Use the mass spectrum below to fill out the information in the table about each isotope.
| Isotope | Proton | Neutron | Mass (amu) | Relative Abundance (%) |
|---|---|---|---|---|
*** SEE BELOW FOR WORKED OUT SOLUTION ***
| Isotope | Proton | Neutron | Mass (amu) | Relative Abundance (%) |
|---|---|---|---|---|
| Strontium-88 | 38 | 50 | 88 | 82.58% |
| Strontium-87 | 38 | 49 | 87 | 7.00% |
| Strontium-86 | 38 | 48 | 86 | 9.86% |
| Strontium-84 | 38 | 46 | 84 | 0.56% |
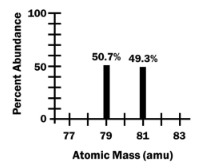
Try it yourself …
Based on the information show on the right,
(a) what is the most likely identity of this element?
(b) Fill in the table below.
| Mass Number | Protons | Neutrons |
|---|---|---|
| 79 | ||
| 81 |